Cerdanyola del Vallès, 27th November 2017. Although carbon monoxide can be fatal, it has been proved that its release in small controlled doses can be beneficial because of its anti-inflammatory, vasodilator and cardio protective properties. Now, new systems developed by researchers from the Universitat Autònoma de Barcelona (UAB), with the collaboration of Research Institute of the Hospital de la Santa Creu i Sant Pau and the ALBA Synchrotron, have been demonstrated useful for transporting molecules that release carbon monoxide when are irradiated with visible light or with ultraviolet.
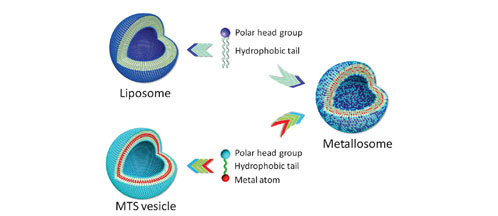
Metalosomes are vesicular aggregates made of phospholipids and metalotensioactives (molecules that have one part with affinity to water, another area that repels water and a metal) with a size less than 1 micron. Those vesicles, as well as other structures that are obtained using different proportions of the two constituent molecules (such as micelles or bicelles), are stable at dilution, meaning that they can be directed to the areas where the effect of the carbon monoxide is needed. In comparison with other metal compounds, the toxicity of the metalosomes has been proved between 10 and 20 times lower and, therefore, they are suitable for medical applications.
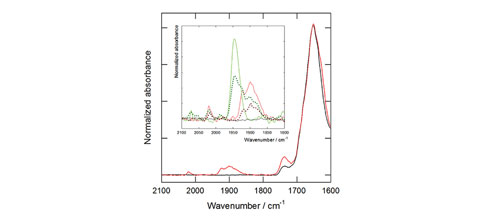
At the MIRAS beamline of the ALBA Synchrotron researchers have analyzed how these systems interact with cells. Using the infrared microspectroscopy, they were able to identify the compounds that were present at the cells, demonstrating their capability as a release system.
This research, which is the result of Maribel Marín-García's doctoral thesis that has been recently defended, paves the way for using mixed systems of phospholipids and metal-surfactants in medical applications and, more specifically, the use of carbon monoxide as a therapy in a controlled and directed delivery system.
Fig 1: Scheme of how metallosomes are built. Fig 2: Infrared spectra of fixed cell cultures of fibroblasts after incubation with a cell culture medium (black) or TCO/SPC 1000 : 3000 mM/mM metallosomes (red) for 12 h. Inset: Details of the CO absorption range for cultures incubated with TCO/SPC 1000 : 3000 mM/mM metallosomes (red); TCO/SPC 1000 : 500 mM/mM small aggregates (dotted dark red); PCO/SPC 1000 : 3000 mM/mM metallosomes (green); and PCO/SPC 1000 : 300 mM/mM small aggregates (dotted dark green).


The group of researchers at the MIRAS beamline in the ALBA Synchrotron. Fom left to right, in the photo group, Joan Suades, Maribel Marín-García, Núria Benseny, Ramon Barnadas and Mercedes Camacho.