Cerdanyola del Vallès, 13th March 2020 As temperatures go up and down, buildings deteriorate, batteries last lesser, and seawater sediments release or store large amounts of methane. All of them are the result of freezing/melting processes, which can be expressed with a simple physics equation.
However, when the same processes are replicated in a lab, Nature’s complexity stubbornly arises.
Scientists have been trying to solidify natural gas for 30 years now. And, as a team lead by the University of Alicante have just found, water-saturated activated carbon can reach a 100% water-to-solidified methane yield, if the right conditions are met. This means that the host material can store over 80% of its weight in the form of methane hydrate, which represents the best outcome for high-pressure methane storage to date. These results, which include ice and methane hydrate measurements at the ALBA Synchrotron, have just been published in the journal Carbon.
The need of solidified natural gas
Natural gas was once thought to be a green fossil fuel. And while concerns have been raised about how clean it truly is, it’s value as the bridge fuel awaiting more efficient renewable energies is still on the rise.
The idea of solidified natural gas was inspired by methane hydrates ––an ice-like solid where a methane molecule is surrounded by water molecules–– stored deep in the oceans. Thus, turning this gas into small pellets that can be easily stored and transported has been a quest for many years. And while activated carbon had already been suggested to be a promising material to store methane, now researchers have demonstrated that under high-pressure conditions this process takes place with an optimal yield.
Water freezing/melting conditions in activated carbon
In this work, scientists first studied samples of activated carbon with different water loads. For undersaturated samples, where adsorbed water is confined in the nano-cavities created to increase the surface area of the host material, a freezing transition was never observed, even when cooled down to temperatures dozens of degrees below the bulk water freezing point. In fact, this shift is predicted in models that take into account the more ordered structure of nano-confined water.
This scenario changed dramatically for samples with larger water loads, where confined water froze at temperatures only around 25 degrees below standard freezing conditions. And more surprising was the behaviour of oversaturated samples, where excess water froze at higher temperatures than bulk water, whereas confined water kept a lower freezing point.
A right amount of adsorbed water as an efficient trigger of methane hydrates
In the quest for efficient solidified natural gas, researchers also evaluated freezing/melting transitions of water-saturated and oversaturated samples under high-pressure methane.
These results showed that an optimal water-to-hydrate conversion can be achieved in saturated and low oversaturated activated carbon for 8.0 MPa high-pressure methane. The 100% yield confirmed this carbon material to be the most efficient to date for the formation of solidified methane. For lower pressure methane environments and/or larger water loads, the water-to-hydrate transition was found to be less efficient, and either confined or excess water remained.
Measurements at ALBA allowed the study of water-to-ice and water-to-hydrate formation
A cooling scan using X-ray powder diffraction at MSPD beamline of the ALBA Synchrotron was used to determine ice and hydrate formation and allowed for the first time the identification of the ice crystals formed when confined water freezes.
During the freezing of the saturated sample, well-defined hexagonal ice molecules were present at the highest temperatures and, surprisingly, some of these were also found at lower temperatures, at which only cubic ice had been reported before.
Under the presence of high-pressure methane, the diffraction pattern ruled out the possibility of a liquid-to-frozen water transition as well as the presence of non-freezable water. At the same time, typical signatures of the methane hydrate formation became visible, which proved the complete water-to-hydrate conversion.
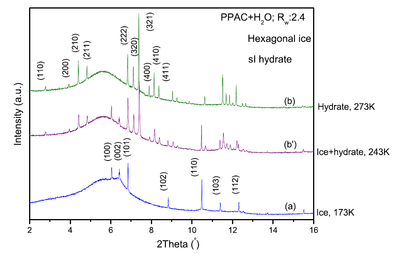
In the image above, the peaks show patterns for a total water-to-ice conversion (blue) and total and partial water-to-hydrate conversion (green and purple) for a saturated sample. More details are given below.
Reference: Freezing/melting of water in the confined nanospace of carbon materials: Effect of an external stimulus. Carlos Cuadrado-Collados, Ahmad A.A. Majid, Manuel Martínez-Escandell, Luke L. Daemen, Aleksandr Missyul, Carolyn Koh, Joaquin Silvestre-Albero. Carbon (2020) 158, 346-355.






